What role does methylation play in autism? Abnormal DNA methylation patterns can disrupt gene functions essential for neural development, contributing to methylation autism spectrum disorder (ASD). Additionally, the role of germ cells in DNA methylation during embryonic development highlights the sensitivity of these cells to environmental factors, which can influence epigenomic changes and affect future generations. This article explores the impact of these methylation changes on autism, investigates environmental factors that influence methylation, and looks at potential treatments targeting these epigenetic changes.
Introduction
Methylation is a crucial biochemical process that plays a vital role in various cellular functions, including gene expression, DNA replication, and epigenetic regulation. It involves the addition of a methyl group to DNA, histones, or other molecules, which can alter gene expression and cellular behavior. In this article, we will delve into the methylation process, its role in gene expression, and the importance of the MTHFR gene in methylation.
Key Takeaways
Methylation is a vital biochemical process that regulates gene expression by modifying DNA accessibility, affecting functions such as neural development and contributing to disorders like autism.
Distinct methylation patterns in individuals with Autism Spectrum Disorder (ASD) suggest that abnormal gene expression linked to neural development is influenced by both genetic and environmental factors.
Potential therapeutic approaches targeting methylation, including pharmacological interventions and dietary supplements, aim to correct abnormal patterns and improve symptoms associated with autism.
Understanding Methylation and Its Role in Gene Expression
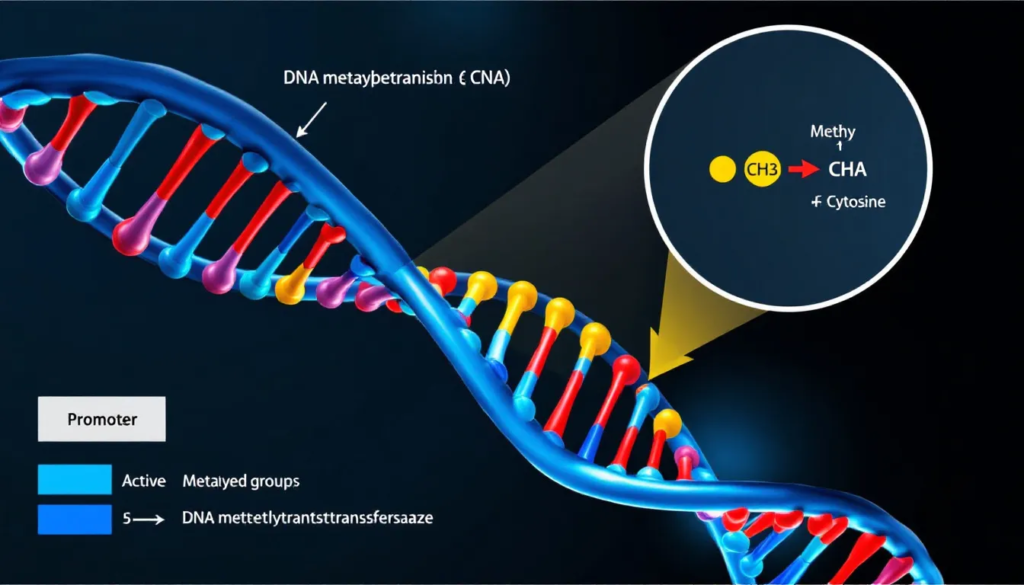
Methylation is a biochemical process that involves the addition of methyl groups to various molecular targets, including DNA. This process is essential for regulating DNA and histones, playing a crucial role in gametogenesis, embryonic growth, and epigenesis. In essence, methylation serves as a fundamental mechanism for controlling gene expression.
During embryonic development, germ cells undergo significant epigenetic changes, and their sensitivity to environmental factors can lead to alterations in DNA methylation patterns that affect future generations.
DNA methylation refers to the conversion of cytosine residues to 5-methylcytosine in the genome, a process catalyzed by DNA methyltransferase enzymes. This modification typically occurs at CpG dinucleotides, where a cytosine nucleotide is followed by a guanine nucleotide. The addition of methyl groups to cytosine results in transcriptional repression, modifying gene expression.
Methylation has an inverse relationship with transcriptional activity. Regions of the genome that are hypomethylated, or have fewer methyl groups, are often associated with active genes that are more accessible for transcription. Conversely, increased methylation typically suppresses gene expression, illustrating the delicate balance maintained by this epigenetic mechanism.
Histone modifications also interact with DNA methylation to influence transcriptional activity. Histones are proteins around which DNA is wound, and their modification can either enhance or suppress gene expression. For example, histone methylation can work in tandem with DNA methylation to reinforce the silencing of specific genes.
Transcription factors, which are proteins that bind to specific DNA sequences to regulate gene expression, can protect certain regions from methylation, including transcription factor binding sites. This protection ensures that essential genes remain active and are not silenced by methylation. Additionally, methyl-binding proteins can bind to methylated DNA regions, recruiting other factors to repress gene expression.
The dynamic nature of DNA methylation allows it to change during development, influencing tissue-specific gene expression patterns. This flexibility is crucial for normal development and function. For instance, during early embryonic development, global methylation patterns undergo significant changes to establish the epigenetic landscape for different tissues and organs.
In summary, DNA methylation is a versatile and dynamic process that plays a vital role in regulating gene expression. By adding methyl groups to DNA, it can modify the accessibility of genes, thereby influencing their activity. This intricate system of epigenetic control is essential for normal development and function, but when it goes awry, it can lead to various disorders, including autism.
Transitioning from the fundamental understanding of methylation, let’s explore how these patterns manifest in individuals with Autism Spectrum Disorder (ASD).
The Methylation Process
Methylation is a complex process that involves the transfer of a methyl group from a donor molecule to a target molecule, such as DNA or histones. This process is catalyzed by enzymes called methyltransferases, which are specific to different types of methylation. DNA methylation, for example, is catalyzed by DNA methyltransferases (DNMTs), which add methyl groups to cytosine residues in DNA. Histone methylation, on the other hand, is catalyzed by histone methyltransferases, which add methyl groups to histone proteins.
DNA methylation typically occurs at CpG dinucleotides, where a cytosine nucleotide is followed by a guanine nucleotide. The addition of methyl groups to cytosine residues results in transcriptional silencing, thereby regulating gene expression. This process is essential for maintaining cellular identity and function, as it ensures that genes are expressed in a tissue-specific manner.
Histone methylation also plays a significant role in regulating gene expression. Histones are proteins around which DNA is wound, and their modification can either enhance or suppress gene expression. Methylation of histones can lead to the formation of heterochromatin, a tightly packed form of DNA that is transcriptionally inactive, thereby contributing to the regulation of gene expression.
Methylation Patterns in Autism Spectrum Disorder (ASD)

Research indicates that individuals with autism display distinct methylation patterns across their genomes compared to those without the disorder. These differences in DNA methylation can affect the regulation of genes involved in neural development and immune responses. Specific genes that are hypermethylated in autistic individuals play crucial roles in neural development and immune responses.
Moreover, alterations in the expression of DNA methyltransferases, such as DNMT3A and DNMT3B, have been linked to neurodevelopmental conditions, including autism. These enzymes are vital for brain function and development, highlighting their importance in understanding the epigenetic landscape of ASD.
Understanding how methylation influences genes linked to autism requires examining the epigenetic regulation of these genes. Additionally, we will explore the environmental factors that can influence methylation patterns and contribute to the risk of ASD.
Epigenetic Mechanisms Regulating Genes Linked to Autism
Epigenetic mechanisms, such as DNA methylation, play a crucial role in regulating gene expression. In autism, these mechanisms can create abnormal methylation patterns impacting gene function related to the disorder. Fetal exposure to endocrine disruptors may alter germline epigenome transmission, causing severe pathologies for multiple generations. Germ cells, which are highly sensitive to environmental factors, play a crucial role in this process. These cells are particularly susceptible to methylation alterations during embryonic development, which can have significant implications for future generations.
Differential methylation can influence gene transcription, with certain genes becoming either overactive or repressed. This imbalance can disrupt normal neural development and contribute to the symptoms of autism. Promoter methylation, in particular, is a key regulator of transcription, as it can silence genes that should be active or activate genes that should be suppressed.
Histone methylation also plays a significant role in epigenetic gene regulation. Modification of histone proteins enables cells to either enhance or suppress the expression of specific genes. This process works in conjunction with DNA methylation to maintain the proper balance of gene activity.
Epigenetic reprogramming, which involves resetting the methylation patterns of the genome, including de novo methylation, is another crucial aspect of gene regulation. This process can occur during development or in response to environmental factors, leading to changes in gene expression that can have lasting effects.
The concept of epigenetic inheritance further illustrates how methylation patterns can be passed down through generations. Changes in the epigenome can be inherited, potentially influencing the risk of autism in offspring.
Global methylation patterns, which refer to the overall methylation status of the genome, can also provide insights into the epigenetic regulation of genes linked to autism. Abnormal global methylation can disrupt the delicate balance of gene expression, leading to neurodevelopmental disorders.
Imprinted genes, which are genes that are expressed in a parent-of-origin-specific manner, are another area of interest. These genes can be particularly sensitive to methylation changes, and their dysregulation can contribute to autism.
RNA interference, a process that involves the regulation of gene expression by small RNA molecules, is also influenced by methylation patterns. This interaction highlights the complexity of epigenetic control and its impact on gene expression.
In summary, the epigenetic regulation of genes linked to autism involves a complex interplay of DNA methylation, histone modifications, and other mechanisms. These processes can lead to abnormal gene expression, contributing to the development and symptoms of autism. Understanding these mechanisms is crucial for developing potential therapeutic approaches.
Next, let’s explore the environmental factors that can influence methylation patterns and contribute to the risk of ASD.
Environmental Factors Influencing Methylation in ASD
Environmental factors, such as exposure to certain chemicals, are believed to modify DNA methylation patterns, influencing the risk of autism. Exposure to lead, for example, has been linked to changes in DNA methylation associated with neurodevelopmental disorders, including autism. Polychlorinated biphenyls (PCBs) have also been linked to alterations in DNA methylation in the developing brain, which may contribute to autism risk. Similarly, exposure to environmental toxins during pregnancy, such as tobacco and pollutants, can significantly affect DNA methylation patterns in developing fetuses.
Maternal factors such as obesity and stress have been associated with alterations in fetal methylation profiles, which may contribute to the risk of autism. Maternal health and environmental exposures during pregnancy significantly influence neurodevelopment, as highlighted by these findings.
The dynamic nature of DNA methylation suggests that it can be influenced by various environmental stresses, impacting gene expression and potentially contributing to autism. This flexibility allows the epigenome to respond to external factors, leading to changes in gene activity that can have lasting effects.
Moreover, certain environmental chemicals have been identified as potential risk factors for autism by impacting DNA methylation and neurodevelopment. These chemicals can disrupt normal methylation patterns, leading to abnormal gene expression and increased risk of neurodevelopmental disorders.
The influence of environmental factors on methylation patterns underscores the importance of considering both genetic and environmental elements in understanding autism. By studying these interactions, researchers can gain insights into the complex causes of ASD and develop strategies to mitigate risk.
In the next section, we will delve into the role of DNA methyltransferases in neural development and their significance in autism.
DNA Methyltransferases and Their Role in Neural Development
DNA methyltransferases play a crucial role in establishing and maintaining DNA methylation patterns across the genome. These enzymes ensure the proper functioning of DNA methylation, which regulates gene expression. The DNA methyltransferase family includes key enzymes such as DNMT1, DNMT3A, and DNMT3B, which are vital for establishing and maintaining DNA methylation patterns during early development.
DNMT3A and DNMT3B are primarily responsible for creating novel methylation patterns, especially during the differentiation of stem cells. These enzymes are crucial for the proper development of the brain and other tissues. Knockout models of DNMT3A and DNMT3B reveal that these enzymes are essential for proper neuronal differentiation and maturation. Without them, the development of the nervous system is severely impaired.
During neural development, DNMT3A plays a significant role in regulating neurodevelopmental genes, influencing processes such as synaptic plasticity and learning. This enzyme ensures that the genes necessary for brain function are appropriately methylated and expressed. The precise regulation of these genes is crucial for the development of cognitive abilities and behavioral responses.
Enzymes associated with methylation, such as MECP2 and UBE3A, play critical roles in the development of autism when mutated. Mutations in these enzymes can lead to abnormal methylation patterns, disrupting the normal function of neurodevelopmental genes. This disruption can contribute to the development of autism and other neurodevelopmental disorders.
The role of DNA methyltransferases in neural development highlights the importance of proper methylation regulation for brain function. By establishing and maintaining methylation patterns, these enzymes ensure that the genes necessary for neural development and function are appropriately regulated.
The interplay between genetic and environmental factors in autism will be examined next, focusing on how these elements influence ASD risk factor.
The Role of MTHFR in Methylation
The MTHFR gene plays a crucial role in methylation by encoding the enzyme methylenetetrahydrofolate reductase (MTHFR). This enzyme is involved in the conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, which is necessary for the synthesis of methionine and the maintenance of DNA methylation. MTHFR gene polymorphisms, such as the 677C>T and 1298A>C variants, can affect the activity of the MTHFR enzyme and lead to changes in DNA methylation patterns. These changes can, in turn, affect gene expression and increase the risk of various diseases, including cancer, cardiovascular disease, and neurological disorders.
MTHFR gene polymorphisms have been associated with changes in DNA methylation patterns, including promoter methylation and gene silencing. For example, studies have shown that individuals with the 677C>T polymorphism have increased levels of homocysteine and decreased levels of methionine, which can lead to changes in DNA methylation patterns and gene expression. Additionally, MTHFR gene polymorphisms have been linked to an increased risk of neural tube defects, such as spina bifida and anencephaly, which are thought to be caused by changes in DNA methylation patterns during embryonic development.
In conclusion, methylation is a complex process that plays a crucial role in gene expression and epigenetic regulation. The MTHFR gene is essential for the maintenance of DNA methylation patterns, and MTHFR gene polymorphisms can affect gene expression and increase the risk of various diseases. Further research is needed to fully understand the role of MTHFR in methylation and its implications for human health.
Interplay Between Genetic and Environmental Factors in Autism
Research indicates that interactions between genetic predispositions and environmental elements are significant in the development of autism spectrum disorders. These interactions can lead to changes in DNA methylation, which in turn affect gene expression and neurodevelopment. These interactions are key to developing comprehensive models of autism.
Environmental factors can induce changes in DNA methylation that affect neuronal stem cell behavior, further implicating DNMTs in responses to external stimuli. For instance, exposure to certain chemicals can modify methylation patterns, leading to abnormal gene expression and increased risk of neurodevelopmental disorders, including autism.
Ongoing studies aim to clarify how early-life environmental factors influence methylation and autism risk. These studies are exploring the impact of various environmental exposures, such as toxins and maternal health, on the development of the nervous system. Understanding these influences allows researchers to develop strategies to mitigate autism risk.
Evidence suggests that the genetic background may enhance vulnerability to the negative effects of environmental factors on neurodevelopment. Certain genetic factors, such as mthfr gene polymorphisms, can influence the effectiveness of dietary interventions in ASD treatment. These findings highlight the importance of considering both genetic and environmental factors in developing personalized treatment plans for autism.
Integrative approaches combining genetic, environmental, and epigenetic data are essential for developing comprehensive models of autism. By considering all these factors, researchers can gain a more complete understanding of the causes of autism and develop more effective strategies for prevention and treatment.
The following section explores potential therapeutic approaches targeting methylation abnormalities in ASD.
Potential Therapeutic Approaches Targeting Methylation
Pharmacological interventions targeting DNA methylation are being investigated as potential treatments for autism spectrum disorder (ASD). These interventions seek to correct abnormal methylation patterns associated with autism, potentially improving symptoms and overall functioning.
Pharmacological agents that inhibit DNA methyltransferases are being explored as potential treatments to address abnormal methylation patterns associated with ASD. Inhibiting these enzymes aims to restore normal methylation patterns and gene expression, potentially alleviating autism symptoms.
Folic acid supplementation has shown promise as a dietary intervention to influence methylation patterns associated with ASD. Folic acid is a vital nutrient for DNA methylation and can help correct abnormal methylation patterns. Studies have shown that supplementation with folic acid during pregnancy may reduce the risk of autism in offspring.
The modulation of epigenetic mechanisms, particularly through methylating agents, is being studied for its potential to correct abnormal gene expression in ASD. These agents can modify methylation patterns, potentially restoring normal gene function and improving symptoms.
Tailored treatment plans based on individual methylation profiles may emerge from insights into methylation research. By understanding the specific methylation patterns associated with each individual, researchers can develop personalized treatment strategies that are more effective in addressing the unique needs of each person with autism.
Potential therapies targeting methylation abnormalities in ASD include pharmacological interventions, dietary supplements, and personalized treatment plans. These strategies aim to correct abnormal methylation patterns and improve symptoms, offering hope for individuals with autism and their families.
The clinical implications of methylation research in autism will be explored next, focusing on how these findings can enhance early diagnosis and treatment.
Clinical Implications of Methylation Research in Autism
Understanding methylation can enhance early autism diagnoses by identifying epigenetic markers. These markers can serve as biomarkers for diagnosing ASD and monitoring therapeutic efficacy. Identifying specific methylation patterns associated with autism enables the development of more accurate diagnostic tools.
Alterations in methylation patterns may serve as biomarkers for diagnosing ASD and monitoring therapeutic efficacy. These biomarkers can provide valuable insights into the underlying causes of autism and help track the effectiveness of treatments.
Advancements in methylation research could lead to the identification of biomarkers for early diagnosis and intervention in autism spectrum disorders. Early diagnosis is crucial for providing timely interventions that can improve outcomes for individuals with autism.
Tailored treatment plans for autism may emerge from insights into individual methylation patterns. Understanding individual methylation profiles enables the development of personalized treatment strategies, addressing the unique needs of each person with autism more effectively.
Clinical trials have shown that folinic acid can lead to improvements in both neurological and behavioral symptoms in children diagnosed with ASD. This finding highlights the potential benefits of dietary supplements in influencing methylation patterns and improving symptoms.
Supplementation with folic acid during pregnancy may reduce the risk of autism spectrum disorder (ASD) in offspring, as indicated by various studies. This finding underscores the importance of maternal health and nutrition in influencing the risk of autism.
Research suggests potential for epigenetic therapies to reduce autism severity through targeted methylation. These therapies aim to correct abnormal methylation patterns, potentially improving symptoms and overall functioning.
The clinical implications of methylation research in autism encompass enhanced early diagnosis, identification of biomarkers, tailored treatment plans, and potential epigenetic therapies. These findings offer hope for improving the lives of individuals with autism and their families.
The future directions in methylation and autism research, including emerging technologies and potential breakthroughs, will be explored next.
Future Directions in Methylation and Autism Research
Emerging sequencing technologies are anticipated to enhance the understanding of dna sequence and DNA methylation patterns associated with autism. These technologies offer detailed insights into the epigenetic landscape, helping researchers identify specific methylation changes associated with autism.
Mass spectrometry is being explored as a tool for precise analysis of methylation, potentially leading to more detailed insights into autism-related epigenetics. This technique can provide high-resolution data on methylation patterns, helping researchers identify subtle changes that may contribute to autism.
Epigenetic reprogramming research may yield breakthroughs in understanding environmental influences on autism. Epigenetic reprogramming involves resetting the methylation patterns of the genome, potentially reversing the effects of environmental exposures and reducing the risk of autism.
The future of methylation research in autism is promising, with emerging technologies and innovative approaches offering new insights and potential breakthroughs. Exploring the connections between methylation and autism further enables the development of more effective strategies for prevention, diagnosis, and treatment.
As we conclude this comprehensive guide, let’s summarize the key points and reflect on the potential impact of understanding methylation in autism.
Summary
In summary, methylation is a crucial biochemical process that regulates gene expression by adding methyl groups to DNA. This process plays a vital role in normal development and function, but abnormal methylation patterns can contribute to various disorders, including autism.
Individuals with autism display distinct methylation patterns that affect genes involved in neural development and immune responses. Environmental factors, such as exposure to chemicals and maternal health, can modify DNA methylation patterns, influencing the risk of autism.
DNA methyltransferases, such as DNMT3A and DNMT3B, play a crucial role in establishing and maintaining methylation patterns during neural development. These enzymes are essential for the proper functioning of neurodevelopmental genes.
The interplay between genetic and environmental factors is significant in the development of autism. By understanding these interactions, researchers can develop comprehensive models of autism and more effective strategies for prevention and treatment.
Potential therapeutic approaches targeting methylation abnormalities in ASD include pharmacological interventions, dietary supplements, and personalized treatment plans. These strategies offer hope for improving symptoms and overall functioning.
The clinical implications of methylation research in autism include enhanced early diagnosis, the identification of biomarkers, tailored treatment plans, and the potential for epigenetic therapies. These findings offer hope for improving the lives of individuals with autism and their families.
As we look to the future, emerging technologies and innovative approaches promise to enhance our understanding of methylation and autism. By continuing to explore these connections, researchers can develop more effective strategies for prevention, diagnosis, and treatment.
Frequently Asked Questions
What is DNA methylation?
DNA methylation is the process of adding methyl groups to DNA, which generally represses gene expression and activity. This modification plays a significant role in regulating various biological processes.
How does methylation relate to autism?
Methylation is linked to autism through unique patterns affecting genes associated with neural development and immune responses. This suggests a potential role of epigenetics in the condition.
Can environmental factors influence methylation in autism?
Environmental factors, including exposure to certain chemicals and aspects of maternal health, can indeed influence DNA methylation patterns, which may affect the risk of autism. Such modifications highlight the complex interplay between genetics and the environment in the development of this condition.
Are there therapeutic approaches targeting methylation in autism?
Yes, pharmacological interventions and dietary supplements such as folic acid are being investigated as therapeutic approaches to target abnormal methylation patterns in autism.
What are the clinical implications of methylation research in autism?
Methylation research has significant clinical implications, as it can improve early diagnosis, identify potential biomarkers, and facilitate the development of personalized treatment plans for individuals with autism.

